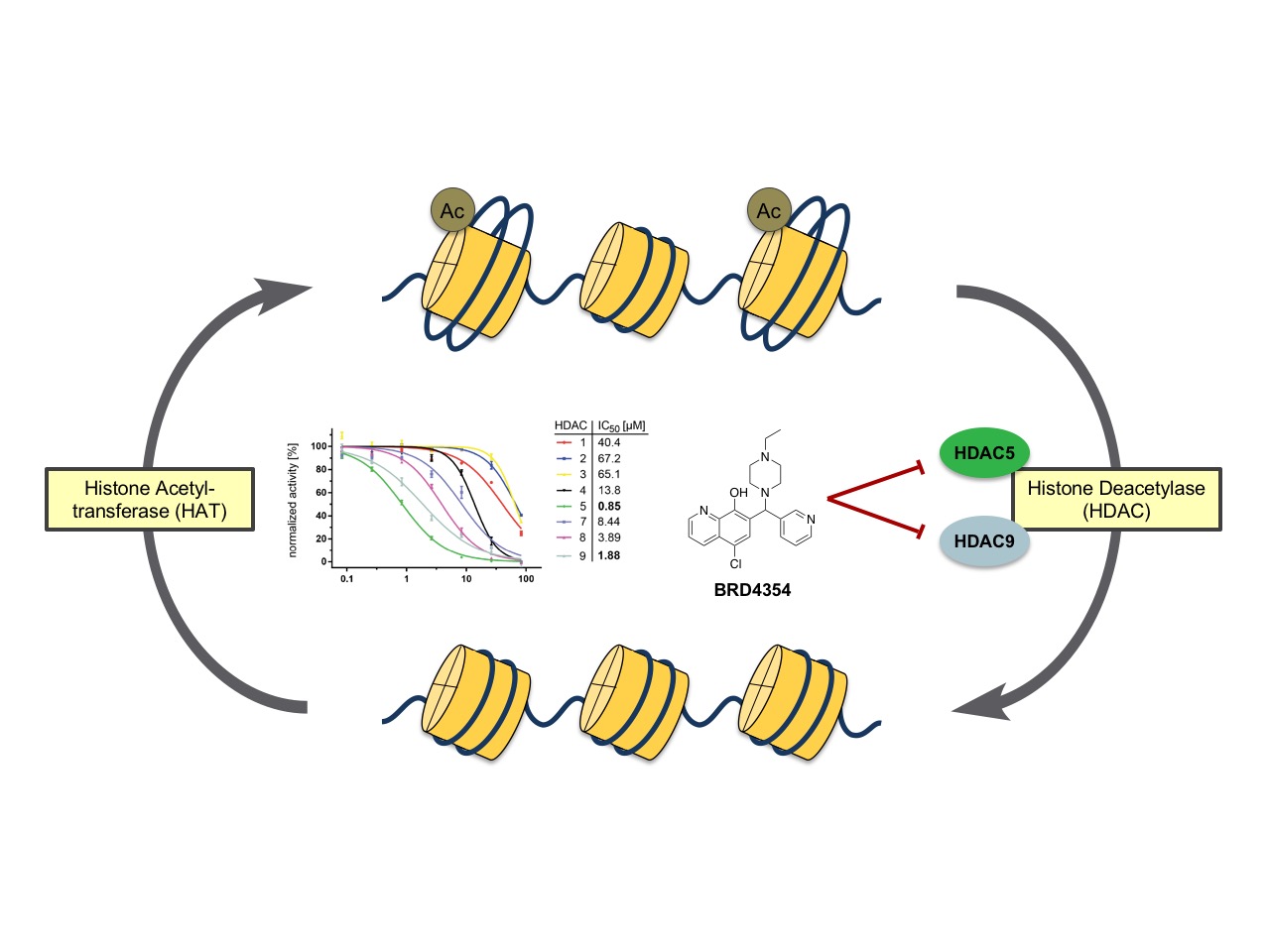
The zinc-dependent histone deacetylase family (HDAC) of proteins regulate a variety of cellular functions by deacetylation of lysine residues that govern protein function for a wide spectrum of protein substrates. Based on sequence homology, the family is grouped into four subclasses which are thought to be somewhat definitive of biological function. Chemical probes that are specific to certain members of the HDAC family can help to elucidate their unique biological activities in a cellular context. BRD4354, a small molecule that shows specificity for modulation of HDAC5 and HDAC9, was discovered using Small Molecule Microarray (SMM) technology. The compound shows inhibition of deacetylase activity of HDACs 5 and 9 with IC50values of 0.85 µM and 1.88 µM, respectively. The mechanism of HDAC inhibition with BRD4354 is thought to be a zinc-dependent modification of specific cysteine residues of the protein (ESI/MS). ACS Chem. Biol., 2016, 11(7), 1844-1851.